The Phase III clinical study conducted in China of TLX250-CDx, a global innovative radionuclide-drug conjugate (RDC) for the diagnosis of clear cell renal cell carcinoma (ccRCC), has completed the first patient enrollment and dosing recently.
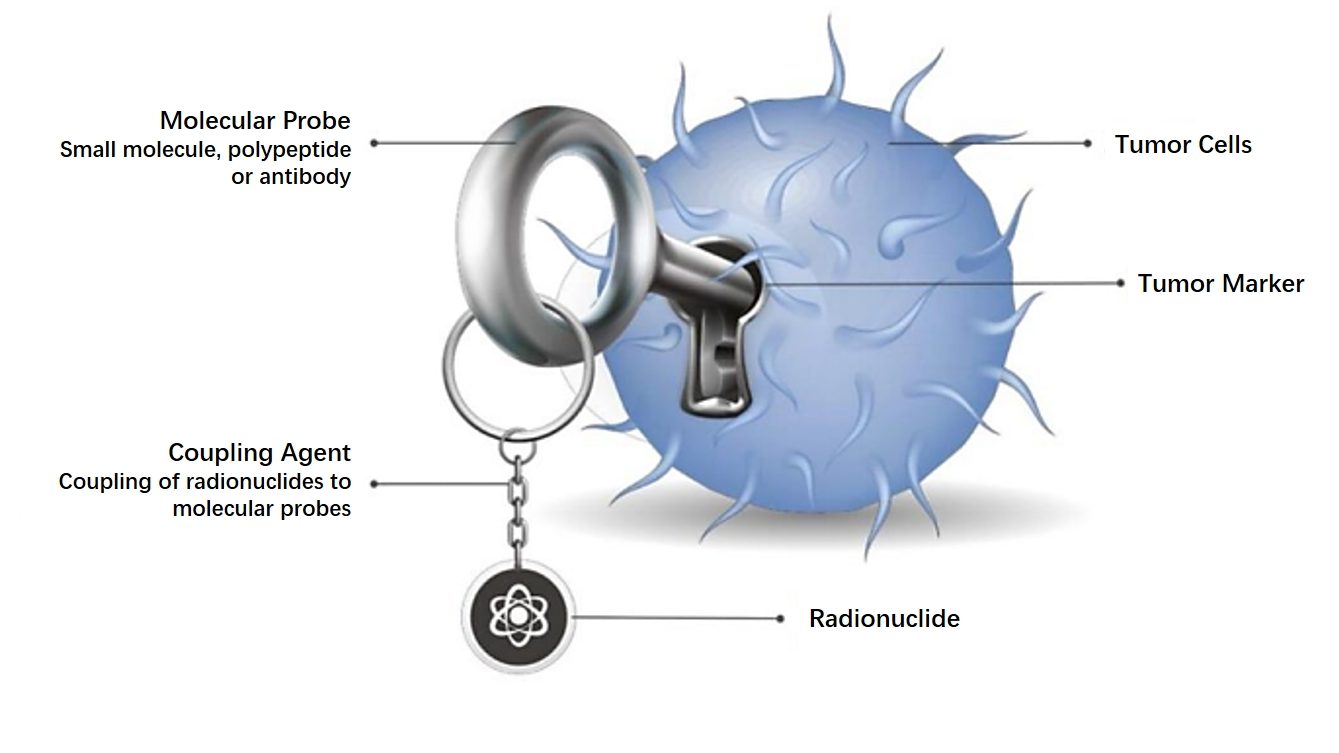
TLX250-CDx (89Zr-TLX250) is a globally innovative radionuclide conjugated drug for the diagnosis of ccRCC. Its target is carbonic anhydrase IX (“CAIX”). CAIX is overexpressed in ccRCC and various cancer types. TLX250-CDx was granted Breakthrough Therapy Designation by the US Food and Drug Administration (FDA) in July 2020 based on its important breakthroughs in non-invasive diagnosis for the most common and aggressive type of kidney cancer – ccRCC as well as the patients’ follow-up treatment and management decisions.
By adhering to the treatment concept of integrated oncology diagnosis and treatment, the Group has reserved 12 innovative products in its nuclear medicine anti-tumor diagnosis and treatment segment, including 5 radionuclides including 68Ga, 177Lu, 131I, 90Y, and 89Zr, and covering 7 cancers including liver cancer, prostate cancer and brain cancer. In terms of product types, it covers two types of radionuclide drugs for diagnosis and therapy, providing patients with multi-indication treatment options, multi-methods and integrated diagnosis and treatment of the world’s leading anti-tumor solutions.