Grand Pharmaceutical Group Limited (0512.HK, Grand Pharma, together with its subsidiaries, the Group) has entered into a strategic cooperation agreement for product introduction with LianBio Development (HK) Limited and Tarsus Pharmaceuticals, Inc.. After the relevant conditions are met, the Group will acquire the exclusive development, production and commercialization rights in Greater China Region (Mainland China, Hong Kong Special Administrative Region of China, Macau Special Administrative Region of China, and Taiwan Region) for TP-03, a global innovative ophthalmic preparation for the potential treatment of Demodex blepharitis and Meibomian Gland Disease (MGD) in patients with Demodex mites with an upfront payment of USD15 million and a certain amount of registration milestone fees. This strategic cooperation will deepen the strategic plan of the Group’s products in the field of ophthalmology.
TP-03 is a non-competitive antagonist selective for gamma-aminobutyric acid-gated chloride channels (“GABA-Cl”). By selectively inhibiting GABA-Cl in Demodex mites, TP-03 paralyzes and kills the mites, which are the root cause of Demodex blepharitis. In addition, TP-03 is highly lipophilic, which promotes its absorption into the oils of eyelash follicles where mites reside. TP-03 has completed two pivotal clinical studies in the United States, collectively involving more than 800 patients with Demodex blepharitis. According to the clinical results, both trials met the primary endpoint and all secondary endpoints, with statistical significance and no serious treatment-related adverse events. The product was approved for commercialization by the United States Food and Drug Administration (“FDA”) in July 2023. It is the first and only drug approved by the FDA for Demodex blepharitis. In addition, there are positive topline results of TP-03 Phase II clinical research in the United States for the treatment of MGD patients with Demodex mites.
In terms of registration in China, TP-03 has completed a Phase III clinical study. The results show that compared with the control group, TP-03 has a statistically significant eradication rate of Demodex infection in patients with Demodex blepharitis (p<0.001) and the cure rate of eyelid cuff discharge also showed a positive but not statistically significant trend (p=0.15). Additionally, TP-03 was well tolerated, and its safety characteristic was similar to that observed in other large-scale clinical trials, with no treatment-related discontinuations. After the completion of this transaction, the Group will vigorously work toward the registration and implementation of TP-03 in China to benefit patients with Demodex blepharitis as soon as possible.
Blepharitis is a common ophthalmic disease that is characterized by eyelid margin
inflammation. Demodex blepharitis is a chronic inflammatory reaction caused by Demodex
infestation of the eyelid margin, which accounts for over two-thirds of all blepharitis cases,
mainly involving the eyelid skin, eyelash follicles and glands, and meibomian glands. Its
typical clinical manifestations include eye itching, foreign body sensation, dry eyes, eyelid
congestion, scales, and cuff-like secretions at the base of the eyelashes. In severe cases, it can
cause conjunctival and corneal complications, and the disease may be contagious to a certain
extent. In addition, Demodex mites are also a risk factor for MGD. MGD patients with
Demodex mites often present with inflammation of the eyelid margin and blurred vision, and
can result in blockage and/or decreased production of meibum liquid. If left untreated, it can
lead to permanent changes to the tear film and progressive gland loss. According to statistics,
there are currently more than 40 million patients with Demodex blepharitis and more than 70
million patients with MGD in China, indicating a large potential patient population.
Currently, there are no drugs that have been commercialized and approved for Demodex
blepharitis in China. There is an urgent clinical need for a safe and effective therapeutic drug
that has rapid effect and can directly act on the root cause of disease, and TP-03 is expected to
fill this clinical gap.
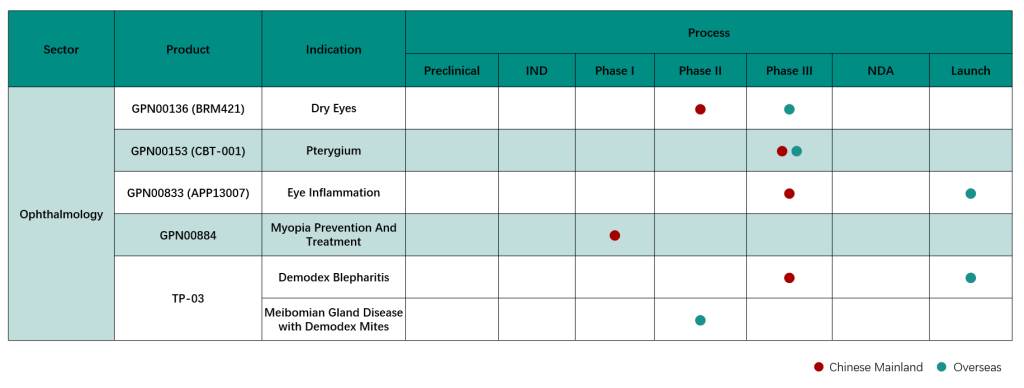
As one of the major Eye, Nose & Throat (“ENT”) drug R&D, production and sales integrated enterprises in China, the number of the Group’s products on sale ranks among the top of the industry. Its treatment areas covering diseases in multiple departments including ophthalmology, otolaryngology, and stomatology, covering chemical preparations, Chinese drug preparations and health products, including prescription drugs, OTC drugs, medical devices, consumer goods and other major categories, covering both channel of inside and outside hospital, create a “great ENT ecosystem” by integrating “prevention + treatment + health care”. In terms of innovation and R&D, the Group has reserved a few world-wide innovative products for the treatment of “myopia”, “dry eye”, “pterygium” and “anti-inflammatory and analgesic after ophthalmology surgery”, and have made significant research and development progress in 2023. Among them, the innovative product CBT-001 for the treatment of pterygium was approved to conduct Phase III clinical study in China in March 2023; GPN00136 (BRM421), a small molecule peptide drug for the treatment of dry eye, was approved to conduct Phase II clinical study in China in April 2023; GPN00833, a hormone nanosuspension eye drops for anti-inflammatory and analgesic, has completed first patient’s enrollment and dosing in Phase III clinical trial in China in October 2023. In terms of overseas registration, the product was approved for commercialization by the FDA in March 2024; GPN00884, a global innovative ophthalmic drug used to delay the progression of myopia in children, was approved to conduct Phase I clinical study in China in March 2024. In the future, the field will adhere to the development strategy of “Chinese and Western combination” and “treatment with both medicines and devices”, continuously strengthen the influence of the industry, and achieve new breakthroughs in the business field.
The Group always puts a high value on the R&D of innovative products and advanced
technologies. Sticking to patients-centered and innovation-driven, the Group will continue to
increase its investment in world-class innovative products and advanced technologies to meet
unmet clinical needs, and enrich product pipeline and improve supply chain. The Group
adopts the strategy of “global expansion and dual-cycle operation”, forming a new pattern of
domestic and international cycles that synergize with each other. In this way, the Group can
make full use of its industrial advantages and R&D capabilities, to accelerate
commercialization process for innovative products and provide patients with more advanced
and diverse treatment options in the world.
Warning
The aforementioned product is pending for registration and approval in the Licensed Region. The approval of commercialization and manufacturing of such products is subject to various factors with uncertainty. Whether the transaction will be profitable is also uncertain. Shareholders and prospective investors of the Company are advised to exercise caution when dealing in the securities of the Company.